QCT and QM calculations of the Cl(2P) + NH3 reaction: influence of the reactant well on the dynamics
Abstract
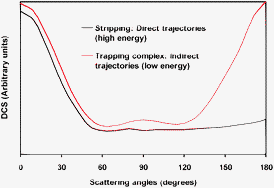
A detailed dynamics study, using both quasi-classical trajectory (QCT) and reduced-dimensional quantum mechanical (QM) calculations, was carried out to understand the reactivity and mechanism of the Cl(2P) + NH3 → HCl + NH2 gas-phase reaction, which evolves through deep wells in the entry and exit channels. The calculations were performed on an analytical potential energy surface recently developed by our group, PES-2010 [M. Monge-Palacios, C. Rangel, J. C. Corchado and J. Espinosa-Garcia, Int. J. Quantum. Chem., 2011], together with a simplified model surface, mod-PES, in which the reactant well is removed to analyze its influence. The main finding was that the QCT and QM methods show a change of the reaction probability with collision energy, suggesting a change of the atomic-level mechanism of reaction with energy. This change disappeared when the mod-PES was used, showing that the behaviour at low energies is a direct consequence of the existence of the reactant well. Analysis of the trajectories showed that different mechanisms operate depending on the collision energy. Thus, while at high energies (Ecoll > 5 kcal mol−1) practically all trajectories are direct, at low energies (Ecoll < 3 kcal mol−1) the trajectories are indirect, i.e., with the mediation of a trapping complex in the entry and/or the exit wells. The reactant complex allows repeated encounters between the reactants, increasing the reaction probability at low energies. The differential cross section results reinforce this change of mechanism, showing also the influence of the reactant well on this reaction. Thus, the PES-2010 surface yields a forward–backward symmetry in the scattering, while when the reactant well is removed with the mod-PES the shape is more isotropic.

 Please wait while we load your content...
Please wait while we load your content...