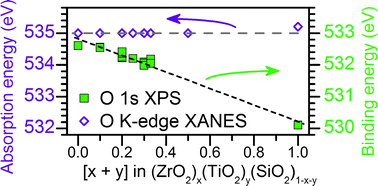
Amorphous quaternary [(ZrO2)x(TiO2)y(SiO2)1−x−y] and ternary [(ZrO2)x(SiO2)1−x] silicates were synthesized using a sol–gel method and examined viaXPS and XANES. Metal silicates are important industrial materials, though structural characterization is complicated because of their amorphous nature. Hard (Ti K- and Zr K-edge) and soft (Ti L2,3-edge) X-ray XANES spectra suggest the Ti and Zr coordination numbers in the quaternary silicates remain constant as the metal identity or total metal content (x, y, or x + y in the chemical formula) is varied. XPS core-line spectra from the quaternary silicates show large decreases in Ti 2p3/2, Zr 3d5/2, Si 2p3/2, and O 1s binding energies due to increasing final-state relaxation with greater next-nearest neighbour substitution of Si for less-electronegative Ti/Zr, which was confirmed by analysis of the O Auger parameter. These decreases in binding energy occur without any changes in the ground-state energies (e.g., oxidation state) of these atoms, as examined by Ti L2,3-edge, Si L2,3-edge, and O K-edge XANES. Because most spectroscopic investigations are concerned with ground-state properties, knowledge of the contributions from final-state effects is important to understand the spectra from materials of interest.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...