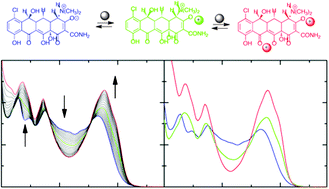
Spectroscopic techniques both in steady-state (in absorption and emission) and pulsed (absorption of excited states with femtosecond resolution) conditions were used to study the complexation process between six molecules belonging to the tetracycline family and Mg2+; in the case of TC the study was extended to the metal ions Ca2+ and Cu2+. The study was carried out in aqueous solution at various pH values, where one acid–base form of the substrate prevails over the others. The processing of experimental results, performed by means of Singular Value Decomposition and Global Analysis methods, allowed us to evaluate the extent of interaction through the association constants, to identify the number of equilibria present in solution and the stoichiometry (1 ∶ 1 or 1 ∶ 2) of the tetracycline ∶ metal ion complex, and to define the spectral and photophysical properties of the latter (in terms of fluorescence quantum yields, lifetimes and rate constants). In fact, the (allowed) radiative decay process is a minor root for the lowest excited state of the complexes which mainly decay to the ground state by internal conversion. Details of the complexation sites are proposed for the various protonated forms of tetracyclines, and for the various cations in the case of TC. In particular, the molecular structure seems to affect significantly the dynamics of interaction when the upper peripheral region of tetracycline is rich in additional hydroxyl groups. Moreover, the state of protonation of the substrate produces changes in the order of the complexation sites, whose affinity for the cation increases significantly when they are negatively charged owing to the loss of protons. Magnesium and calcium (hard cations) give similar interactions, at least in acid solution, while copper(II) (borderline cation) binds more efficiently on different sites, thus forming complexes with different properties.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...