The mathematical origins of the kinetic compensation effect: 2. the effect of systematic errors
Abstract
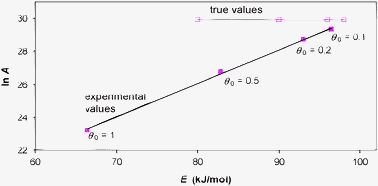
The kinetic compensation effect states that there is a linear relationship between Arrhenius parameters ln A and E for a family of related processes. It is a widely observed phenomenon in many areas of science, notably heterogeneous catalysis. This paper explores mathematical, rather than physicochemical, explanations for the compensation effect in certain situations. Three different topics are covered theoretically and illustrated by examples. Firstly, the effect of systematic errors in experimental kinetic data is explored, and it is shown that these create apparent compensation effects. Secondly, analysis of kinetic data when the Arrhenius parameters depend on another parameter is examined. In the case of

 Please wait while we load your content...
Please wait while we load your content...