An operando Raman study of molecular structure and reactivity of molybdenum(vi) oxide supported on anatase for the oxidative dehydrogenation of ethane
Abstract
Supported ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo(–O–)3 tetra-coordinated species in C3v-like symmetry prevail at all surface coverages with a low presence of associated (polymeric) species (probably penta-coordinated) evidenced at high coverages, below the approximate monolayer of 6 Mo per nm2. A mechanistic scenario for 18O/16O isotope exchange and next-nearest-neighbor vibrational isotope effect is proposed at the molecular level to account for the pertinent spectral observations. Catalytic measurements for
Mo(–O–)3 tetra-coordinated species in C3v-like symmetry prevail at all surface coverages with a low presence of associated (polymeric) species (probably penta-coordinated) evidenced at high coverages, below the approximate monolayer of 6 Mo per nm2. A mechanistic scenario for 18O/16O isotope exchange and next-nearest-neighbor vibrational isotope effect is proposed at the molecular level to account for the pertinent spectral observations. Catalytic measurements for ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band intensities for surface densities of 1.8–5.9 Mo per nm2 and various residence times show that the terminal Mo
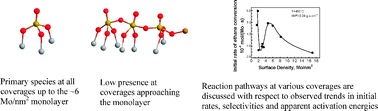
O band intensities for surface densities of 1.8–5.9 Mo per nm2 and various residence times show that the terminal Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O sites are involved in non-selective reaction turnovers. Reaction routes follow primarily non-selective pathways at low coverage and selective pathways at high coverage. Trends in the initial rates of ethane consumption (apparent reactivity per Mo) as a function of Mo surface density are discussed on the basis of several factors.
O sites are involved in non-selective reaction turnovers. Reaction routes follow primarily non-selective pathways at low coverage and selective pathways at high coverage. Trends in the initial rates of ethane consumption (apparent reactivity per Mo) as a function of Mo surface density are discussed on the basis of several factors.
- This article is part of the themed collection: Operando surface spectroscopy

 Please wait while we load your content...
Please wait while we load your content...