Atmospheric hydrocarbonactivation by the hydroxyl radical: a simple yet accurate computational protocol for calculating rate coefficients†
Abstract
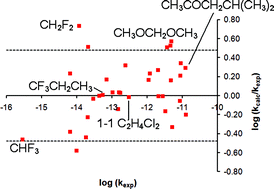
The overall rate coefficient at standard temperature and pressure for the hydrogen abstraction reaction by the hydroxyl radical (HO˙) from common saturated volatile organic compounds (VOCs) is derived theoretically using electronic structure calculations and transition state theory (TST). The computational approach used is based on relatively efficient methods, and hence is applicable to a large number of compounds with only a modest use of computer resources. The key methods used are density functional theory (for the calculation of barrier heights) and simple transition state theory (TST), including a simple correction for tunnelling. All thermally relevant conformers of the reactant and the abstraction TS are included in the study. For all compounds in a test set of thirty-four, the calculated rate coefficient agrees with the experimental value to within better than an order of magnitude, and to within better than a factor of three for all but six cases, so that the accuracy is of predictive utility.

 Please wait while we load your content...
Please wait while we load your content...