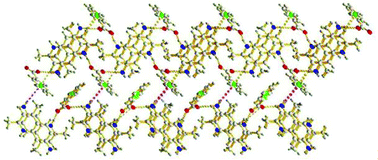
The 7-amino-2,4-dimethylquinolinium salts with a variety of anions (Cl−, HCOO−, CH3COO−, PhCOO−, L-HOOCCH(OH)CH(OH)COO−) have been synthesized and characterized. The crystal structures of these salts were determined by single-crystal X-ray diffraction. The structure analysis confirms that the nitrogen atoms in the quinoline rings are protonated in all salts. The two solvates have been obtained and thereby provide a useful complement to cocrystal screening. All the quinolinium salts display interesting three dimensional supramolecular networks. The hydrogen bonding interactions observed in all of the salts are N–H⋯O, O–H⋯O and N–H⋯Cl, together with weak C–H⋯O, C–H⋯N, C–H⋯Cl hydrogen bonds. The weak C/N–H⋯π contacts and π–π stacking interactions involving the quinoline moieties also exist in quinolinium salts. The observed noncovalent interactions become prominent in stabilizing their crystal packing. The 7-amino-2,4-dimethylquinolinium salts show strong luminescence in the solid state and solution in the range 422–534 nm. The solid-state emission spectra of the quinolinium salts are sensitive to the anion species, and highly dependent on the nature of the stacking interactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...