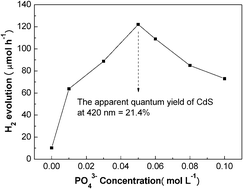
Cubic nanocrystalline CdS was hydrothermally transformed into hexagonal CdS in the presence of Na3PO4 at 180 °C for 12 h. The as-prepared CdS samples were characterized by transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), BET, electrophoretic analysis, photoluminescence (PL) spectra and UV-Vis absorption spectra techniques. Effects of phosphate concentration, hydrothermal time and Pt loading content were investigated. Their photoactivity was evaluated by hydrogen evolution from aqueous solution containing formic acid as a hole scavenger under visible light (λ ≥ 420 nm) irradiation. Phosphate markedly promotes the phase transformation of CdS from cubic to hexagonal. With 0.050 mol L−1 PO43−, the formed hexagonal phase content reaches a maximum (82%). The as-prepared CdS with a high percentage of hexagonal phase displays excellent activity for photocatalytic hydrogen evolution. Pt is highly dispersed on CdS so that the Pt content for the effective hydrogen evolution is very low. The CdS loaded with 0.025 wt% Pt shows the maximum activity for the hydrogen evolution. The apparent quantum yield at 420 nm amounts to 21.4%. This work highlights a facile and low-cost method for the preparation of a highly-efficient CdS photocatalyst. The possible mechanisms were discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...