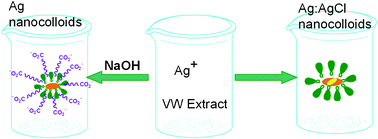
Ag and Ag:AgCl hybrid nanocolloids with excellent stability are selectively synthesized using a room-temperature, aqueous-phase and phyto-reduction approach. The extract of Vernonia anthelmintica (L.) wild seed acts as both a reducer and stabilizer. It also provides a slow-release chlorine resource for the formation of AgCl. A small amount of sodium hydroxide switches the products between Ag and Ag:AgCl nanocolloids. The introduction of NaOH can greatly enhance the reducing ability of organic reducing agents derived from the extract, e.g., phenolics, thus giving rise to complete reduction of AgCl into Ag. Meanwhile, phenolics and vernolic acid-derived carboxylate offer highly effective stabilization to the synthesized nanocolloids. Our approach represents a selective and green synthetic route for Ag-based nanomaterials, and the highly stable Ag and Ag:AgCl nanocolloids should have promising applications in various fields, such as biomedicine and sensors.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...