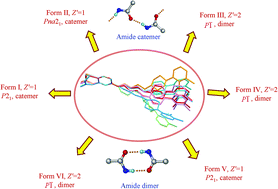
In the present study we report the existence of sixth polymorph of aripiprazole (APPZ) as characterised by single-crystal X-ray diffraction, and present its structural and lattice energy comparison with five other polymorphs of APPZ in the Cambridge Structural Database (CSD). Incidentally, aripiprazole with six well characterised polymorphs happens to be the second most polymorphic system in the CSD after the classic ROY molecule which has a record number of seven polymorphs. The extensive polymorphism in the title compound is attributed to a very high degree of conformational freedom, significant differences in the hydrogen bonding and due to the influence of crystal packing effects. Further, the stabilisation of a metastable conformer by more efficient crystal packing or a less efficient crystal packing by a more stable conformer are believed to contribute its rich polymorphic behavior. Besides these structural features, an interesting observation noted in APPZ system is that, out of the six polymorphs three polymorphs crystallised in centrosymmetric space groups (all P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and the remaining three are in the non-centrosymmetric space groups (P21, P21 and Pna21). The presence or absence of a specific hydrogen bonding motif in the crystal structure is correlated to the selection of the space group. Overall aripiprazole presents an interesting case study of packing, synthon and conformational polymorphism. The present study indicates the importance of exploring suitable crystallisation conditions for a good polymorph screening in the early stages of the drug development.
) and the remaining three are in the non-centrosymmetric space groups (P21, P21 and Pna21). The presence or absence of a specific hydrogen bonding motif in the crystal structure is correlated to the selection of the space group. Overall aripiprazole presents an interesting case study of packing, synthon and conformational polymorphism. The present study indicates the importance of exploring suitable crystallisation conditions for a good polymorph screening in the early stages of the drug development.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and the remaining three are in the non-centrosymmetric space groups (P21, P21 and Pna21). The presence or absence of a specific hydrogen bonding motif in the crystal structure is correlated to the selection of the space group. Overall
) and the remaining three are in the non-centrosymmetric space groups (P21, P21 and Pna21). The presence or absence of a specific hydrogen bonding motif in the crystal structure is correlated to the selection of the space group. Overall 

 Please wait while we load your content...
Please wait while we load your content...