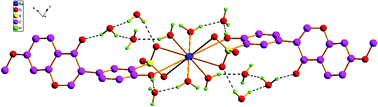
Four novel 3D metal–organic frameworks, [Ba (H2O)4 (L1)2]·4H2O (1), [Ba(H2O)4(L2)2]·8H2O (2) [Ba(H2O)7](L3)2·2H2O (3) and [Ba(H2O)7(L4)2]·3H2O (4) were obtained by self-assembly of barium(II) with four isoflavonesulfonate ligands [L1 = 7,4′-dihydroxyisoflavone-3′-sulfonate, L2 = 4′- hydroxy-7-methoxyisoflavone-3′-sulfonate, L3 = 5-hydroxy-7,4′-dimethoxyisoflavone-3′-sulfonate, L4 = 5,7-dihydroxy-6,4′-dimethoxyisoflavone-3′-sulfonate]. To survey the influences of different substituents in isoflavonesulfonate ligands on their self-assembly manners, the crystal structures of 1–4 have been determined by single-crystal X-ray diffraction. The results show that they have an intriguing variety of coordination modes and stacking manners with the changes of substituents in isoflavonesulfonate ligands. The sulfo-groups of isoflavonesulfonate ligands play important roles, they not only can coordinate with Ba2+ directly, but also provide an important bridge in a structural link between the hydrophilic region and the hydrophobic region in the crystal structure of 1–4.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...