Self-assembled hollow rare earth fluoride alloyed architectures with controlled crystal phase and morphology†
Abstract
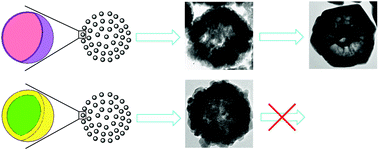
A general synthetic protocol has been developed to assemble EuF3:Ln3+ and EuF3:Ln3+/NH4+ (Ln = Y, Gd, Tb, Dy, Ho, Er, and Tm) alloyed nanocrystallites into hexagon-shaped sub-microcages and hollow sub-microspheres. The structure, morphology and growth kinetics of the alloyed crystallites are investigated. Based on the results obtained by X-ray powder diffraction (XRD), transmission electron microscopy (TEM) and X-ray photoelectron spectra (XPS), it is proposed that the possible formation mechanisms for EuF3:Ln3+ and EuF3:Ln3+/NH4+ (Ln = Y, Gd, Tb, Dy, Ho, Er, and Tm) alloyed architectures involves a crystal-phase-mediated self-assembly process. The crystal structure and morphology are strongly influenced by the Eu/Ln feed molar ratio in the starting solution. A higher feed ratio of Eu to Ln facilitates the growth of hexagonal EuF3:Ln3+ (Ln = Y, Gd, Tb, Dy, Ho, Er, and Tm) alloyed crystallites and results in the formation of hexagon-shaped sub-microcages, while a lower feed ratio of Eu to Ln accelerates the growth of cubic NH4Ln3F10:Eu3+ (Ln = Y, Gd, Tb, Dy, Ho, Er, and Tm) alloyed crystallites and leads to the assembly of the alloyed nanocrystals into hollow sub-microspheres. Additionally, the as-synthesized alloyed architectures show good performance in highly efficient fluorescent host materials.
- This article is part of the themed collection: Nanocrystals

 Please wait while we load your content...
Please wait while we load your content...