Four new cobalt(ii) coordination complexes: thermochromic switchable behavior in the process of dehydration and rehydration†
Abstract
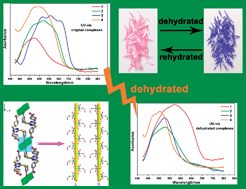
Four cobalt(II) coordination complexes, {[Co11(L1)6(HL1)2(H2O)28]·12H2O}n (1), [Co4(H2L1)4(HL1)2(H2O)8]·2H3L1·2H2O (2), {[Co(H2L3)(H2O)3]·2H2O}n (3) and [Co(HL1)(4,4′-bpy)(H2O)2]n (4), (H3L1 = 2,4,6-tri(3-carboxyphenylthio)-1,3,5-triazine, H4L3 = 2,4-bis(3-carboxyphenylthio)-6-(4-carboxyphenylamino -1,3,5-triazine, 4,4′-bpy = 4,4′-bipyridine), was synthesized hydrothermally and characterized by single-crystal X-ray diffraction, powder X-ray diffraction, IR, thermal analysis, UV-vis spectroscopy and magnetic measurements. Complex 1 shows a 2D layer structure composed of three types of secondary building units (SBUs), [M1], [M3] and [M4] bridged by [HL1]2− and [L1]3− linkers. While complex 2 is a tetranuclear compound and further forms a 3D supramolecular network structure based on eight types of hydrogen-bonding interactions between the molecules. The 2D framework of complex 3 can be described as 1D infinite chains interconnected by [H2L3]2− ligands. Furthermore, the hydrogen-bonding interactions between the adjacent layers along the ac plane makes the 2D layer structure extend to a 3D network. The structure of complex 4 can be illustrated as a 1D zigzag infinite chain with 4,4′-bipyridine as the secondary ligand. Additionally, the π⋯π stacking interactions can be observed between the adjacent chains. An aquo-accessible thermochromic change in complexes 1–4 was induced by means of the reversible dehydration and rehydration of the crystals. Magnetic measurements showed antiferromagnetic coupling between the Co2+ ions in all of the four complexes.

 Please wait while we load your content...
Please wait while we load your content...