Formal C–H amination of cyclopropenes†
Abstract
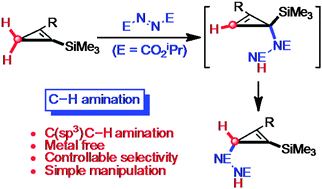
A novel C(sp3)–H amination of trimethylsilyl-substituted cyclopropenes is described. This C–H amination proceeds via a tandem regioselective ene reaction between cyclopropenes and azodicarboxylate to generate a hydrazodicarboxylate intermediate followed by its site-selective allylic transposition.

 Please wait while we load your content...
Please wait while we load your content...