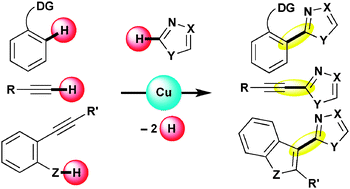
Some new types of copper-mediated intermolecular oxidative direct C–C (hetero)aromatic cross-couplings are described. A combination of the simple CuCl2 salt and molecular oxygen allows 1,3-azoles to couple with terminal alkynes directly to form the corresponding heteroarylacetylenes. This direct version of Sonogashira-type coupling can be applied to the reaction with polyfluoroarenes. A copper acetate complex enables direct biaryl coupling between 2-arylazines and 1,3-azoles even in the absence of any palladium catalysts. Moreover, the Cu-based protocol can be extended to the coupling with indoles and pyrroles, in which a catalytic variant is also possible by using an ideal co-oxidant, atmospheric oxygen. On the other hand, a copper-promoted annulative direct coupling of o-alkynylphenols and -anilines with 1,3-azoles can provide a unique dehydrogenative approach to C3-azolylbenzoheteroles from nonhalogenated and nonmetalated starting materials. In addition, a related N-azolylindole synthesis from similar substrates is also disclosed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...