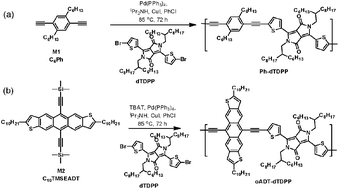
Anthradithiophene was incorporated in a polymer structure by extending its conjugation from the 5,11-positions, through in situ desilylation followed by acetylenic coupling with a dibromo-monomer. The resulting polymer showed largely redshifted order in a thin film as well as order in thin film, forming lamellar structures out of the substrate plane. As a result, it exhibits field-effect hole mobilities, on the order of 0.1 cm2 V−1 s−1, a ten to hundred-fold improvement as compared to previous acene-containing polymers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...