Interplay between an elusive 4-(isopropylamino)imidazol-2-ylidene and its isolable mesoionic tautomer, and associated reactivities†
Abstract
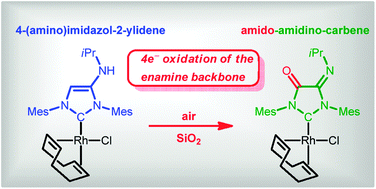
An elusive free 4-(isopropylamino)imidazol-2-ylidene is engaged in a tautomeric equilibrium with its mesoionic tautomer, 4-(isopropylamido)imidazolium, which displays the typical reactivity of a cyclic diaminocarbene; once coordinated to a Rh(I) centre, it undergoes a smooth 4e−oxidation of its backbone to yield an amido–amidino-carbene, a weak

 Please wait while we load your content...
Please wait while we load your content...