Synthesis of tetrahydro-β-carbolines via isomerization of N-allyltryptamines: a metal-catalyzed variation on the Pictet–Spengler theme†
Abstract
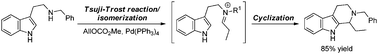
An efficient and broadly applicable alternative to the classical Pictet–Spengler synthesis of tetrahydro-β-carbolines is presented. The method relies on metal-catalyzed isomerization of allylic

 Please wait while we load your content...
Please wait while we load your content...