White phosphorus as a ligand for the coinage metals†
Abstract
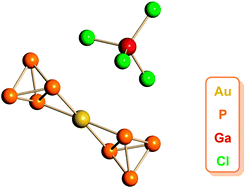
Reaction of equimolar quantities of MX (M = Au, Cu, X = Cl; M = Ag, X = OTf) and GaCl3 in CH2Cl2 with P4 leads to phosphorus ligating a cationic coinage metal centre. For Cu and Ag, ion-contacted coordination

 Please wait while we load your content...
Please wait while we load your content...