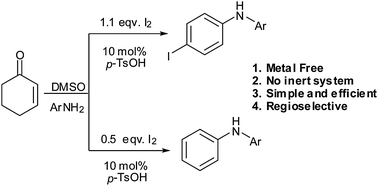
Metal-free direct amination/aromatization of 2-cyclohexenones to iodo-N-arylanilines and N-arylanilines promoted by iodine†
Abstract
An iodine mediated aromatization leading to a one-pot synthesis of iodo-N-arylanilines and N-arylanilines is reported. This highly regioselective aliphatic–aromatic transformation can be performed with various combinations of 2-cyclohexenones and

 Please wait while we load your content...
Please wait while we load your content...