Bisarylindenols: fixation of conformation leads to exceptional properties of photochromism based on 6π-electrocyclization†
Abstract
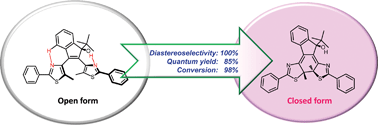
Thermally irreversible photochromic 1-tert-butyl-substituted 2,3-bisthiazolylindenol has been synthesized. It showed perfect diastereoselectivity and high ring-closing quantum yield with high conversion ratio to the closed form. The collaborative interaction of two intramolecular hydrogen bonds and the steric restriction fixed the conformation in favour of

 Please wait while we load your content...
Please wait while we load your content...