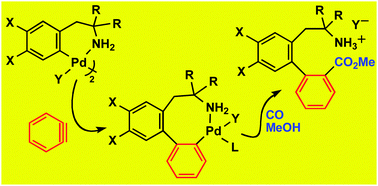
Insertion of benzyne into the Pd–C bond. Synthesis of unnatural amino acid derivatives by sequential insertion of benzyne and CO: 2,2′-functionalized biaryls containing alkylamino and carboxymethyl substituents. Isolation of stable carbopalladated-benzyne intermediates†
Abstract
Reaction of ortho-palladated derivatives of

 Please wait while we load your content...
Please wait while we load your content...