Enantioselective direct aminalization with primary carboxamides catalyzed by chiral ammonium 1,1′-binaphthyl-2,2′-disulfonates†‡
Abstract
A highly effective catalytic enantioselective direct
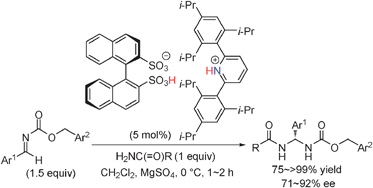
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...