Role of quaternary ammonium salts as new additives in the enantioselective organocatalytic β-benzylation of enals†‡
Abstract
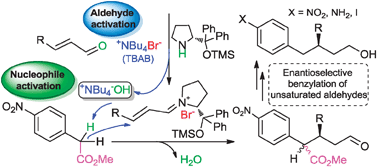
We report herein the efficiency of quaternary ammonium salts as co-catalysts in organocatalytic Michael reactions involving iminium activation of α,β-unsaturated aldehydes. The enantioselective formal benzylation of these substrates has been optimized and used to rationalize the role of the ammonium salts in these processes.
- This article is part of the themed collection: Organocatalysis

 Please wait while we load your content...
Please wait while we load your content...