A highly efficient catalyst-free protocol for C–H bond activation: sulfamidation of alkyl aromatics and aldehydes†
Abstract
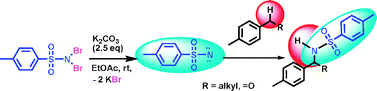
A catalyst-free protocol has been developed for amidation of alkyl aromatics and aldehydes using TsNBr2via a nitrene transfer process in the presence of a base in excellent yield within a short time. The reaction was found to be selective for secondary and tertiary benzylic C–H bonds and C–H bonds of aldehydic groups.

 Please wait while we load your content...
Please wait while we load your content...