Asymmetric transformations involving 1,2-dicarbonyl compounds as pronucleophiles
Abstract
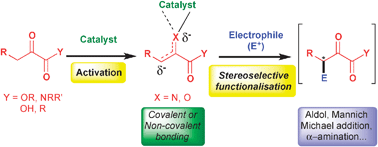
This article concentrates on the versatile nucleophilic reactivity of 1,2-dicarbonyl compounds in various asymmetric transformations. Although underexploited in comparison to their 1,3-dicarbonyl homologues, the presence of adjacent multiple reactive centres allows the selection of specific
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...