Diastereoselective methylation of bis(N-confused porphyrinatonickel(ii)): access to configurationally stable chiral bis(porphyrinoid) and non-symmetric dimers†
Abstract
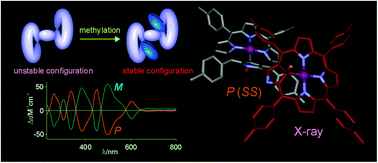
Bis- and tris(methylated) derivatives of 3,3′-bis(N-confused porphyrin) were obtained.

 Please wait while we load your content...
Please wait while we load your content...