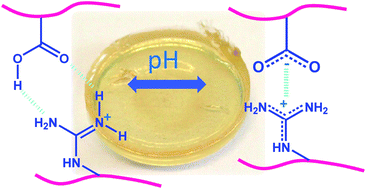
Bio-inspired stabilization of aliphatic polycarbonate-based hydrogels has been carried out by the metal-free ring-opening co-polymerization (ROP) of 6-membered cyclic carbonates containing, respectively, protected guanidine and carboxylic acid functions. Polyethylene glycol (PEG) bound to methylcarboxy trimethylene carbonate at each extremity was used as the cross-linker, and the copolymerizations were performed in CH2Cl2 for 24 h in the presence of 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and N-(3,5-trifluoromethyl)phenyl-N′-cyclohexylthiourea (TU), the catalyst and co-catalyst, respectively. Well-defined hydrogels of various compositions and presenting a high gel fraction were obtained. HR-MAS NMR has been successfully employed to validate our purification technique as well as to assess the selective de-protection of the guanidine and carboxylic acid functions. Evidence of self-assembling properties has been attested by differential scanning calorimetry (DSC), HR-MAS NMR analysis and swelling test experiments in aqueous buffered solutions at pH 4 and 8.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...