Supramolecular organogels based on perylenetetracarboxylic diimide dimer or hexamer†
Abstract
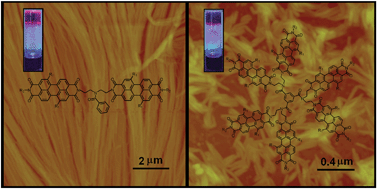
Two new perylenetetracarboxylic diimide (PDI) derivatives, namely dimer 1 and hexamer 2 composed of two or six PDI units respectively, are prepared. The aggregation behaviour of these two compounds in solution was investigated using absorption and

 Please wait while we load your content...
Please wait while we load your content...