Abstract
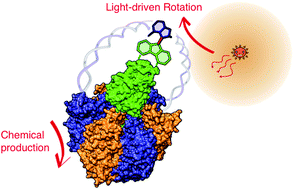
Although a variety of physical perturbations are utilised to control chemical reactions or bias chemical equilibria, mechanical force is not generally an option for controlling chemical reactions due to technical complexity. However, upon attaching handles to a responsive moiety and pulling, twisting or pushing it, unique means of controlling chemistry are permitted because mechanical force is intrinsically anisotropic for chemical structures. One remarkable example in natural systems is F1-ATPase, the water-soluble part of FoF1-ATP synthase. F1-ATPase is a rotary motor

 Please wait while we load your content...
Please wait while we load your content...