Highly enantioselective palladium-catalyzed umpolung allylation of aldehydes†
Abstract
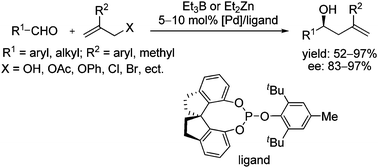
Compared with well-established electrophilic π-allylpalladium chemistry, the catalytic asymmetric reactions via umpolung of π-allylpalladium have received limited success. Although extensive efforts have been devoted, only modest enantioselectivities have been obtained in the

 Please wait while we load your content...
Please wait while we load your content...