A novel stereoselective one-pot synthesis of 2-susbstituted amino-5,6-dihydro-4H-1,3-thiazines via primary allylamines afforded from Morita–Baylis–Hillman acetates†‡
Abstract
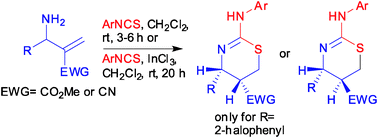
A new stereoselective synthesis of 2-susbstituted amino-5,6-dihydro 4H-1,3-thiazines using primary allylamines obtained from the Morita–Baylis–Hillman (MBH) acetates is described. The primary allylamines react with aryl isothiocyanates to afford the title compounds via sequential thiourea formation and intramolecular sulfa-Michael reaction in a one-pot process under two different experimental conditions. Two steps during the reactions between the allylamines derived from the MBH adducts of

 Please wait while we load your content...
Please wait while we load your content...