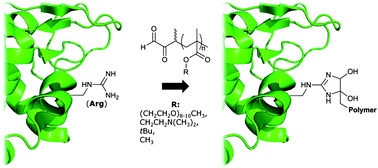
The residue-specific modification of peptides and proteins is a powerful strategy for preparing biomolecular–synthetic polymer conjugates with advanced properties. This manuscript aims at expanding the present toolbox of residue-selective protein modification reactions and targets arginine, a residue for which selective polymer coupling chemistry has only recently been established. To this end, a protected, α-oxo-aldehyde functionalized ATRP initiator that can be used for the preparation of a variety of α-oxo-aldehyde functionalized polymethacrylates has been developed. Polymerization kinetics for four different methacrylate monomers have been investigated in detail and optimized conditions for the chain-end deprotection to reveal the α-oxo-aldehyde end-group have been elaborated. As a final proof of concept, the residue-specific modification of a model protein, chicken egg white lysozyme (HEWL), at arginine residues has been demonstrated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...