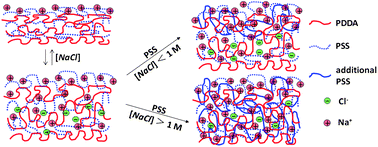
In a two-step process, polyelectrolytes are added to existing layer-by-layer (LbL)-constructed polyelectrolyte multilayer films (PEMs), generating versatile new films as well as fundamental insights into LbL assembly mechanisms. First, PEM swelling is affected by exposure to aqueous salt solutions of high concentration, and second, polyelectrolyte is added to the swollen PEM at the same high salt concentration. Our strategy is illustrated by adding poly(styrene sulfonate) (PSS) to poly(diallyldimethylammonium chloride)/PSS PEMs swollen by NaCl, the rebuilding steps tracked by the quartz crystal microbalance with dissipation, or QCM-D, approach. From the swelling after the first step, the association free energy of polycation and polyanion units is derived, ∼−7.0 kJ mol−1. Swelling by salt strongly affects the rate of attachment, depth of permeation, and rate of diffusion of PSS. At low salt ([NaCl] less than ∼1 M for PEMs constructed at [NaCl] = 0.5 M), PSS is added only at/near the PEM surface, while at high salt ([NaCl] greater than ∼1 M for the same PEMs), PSS fully permeates the PEM, contributing a PSS mass that can approach or exceed that already present; higher salt leads to greater PSS uptake. Even with sizable PSS addition, uptake kinetics is closely diffusive, characterized by surprisingly large and sharply [NaCl]-dependent diffusion coefficients (∼10−14–10−12 cm2s−1). The kinetics at high salt concentreation offer no evidence of slow PEM reorganization, although at low salt concentration, such reorganization remains possible.

 Please wait while we load your content...
Please wait while we load your content...