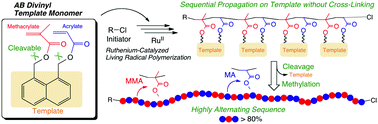
For alternating repeat-unit sequence via living radical polymerization, “template monomers” were designed and polymerized, where two polymerizable alkene (vinyl) functions [e.g., methacrylate (M) and acrylate (A)] were placed side by side at the 1,8-positions on a rigid naphthalene scaffold. Even for such a divinyl monomer, highly selective intramolecular radical propagation was achieved with metal-catalyzed living radical polymerization systems, to give linear controlled polymers without cross-linking. The naphthalene template was cleaved viahydrolysis from the resultant polymer, and subsequently methylated for sequence characterization. 1H NMR analysis demonstrated that the polymers consisted of highly alternating sequences (A-M-A: >80%), practically free from homo triad sequences (M-M-M).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...