Photoinduced decarboxylation of 3-(N-phthalimido)adamantane-1-carboxylic acid and radical addition to electron deficient alkenes†
Abstract
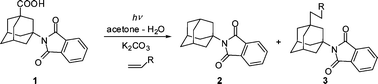
Direct and sensitized excitation of 3-(N-phthalimido)adamantane-1-carboxylic acid (1) leads to the population of the triplet state that, in the presence of a base, decarboxylates, giving

 Please wait while we load your content...
Please wait while we load your content...