Effect of sodium chloride on the binding of polyaromatic hydrocarbon guests with sodium cholate aggregates†‡
Abstract
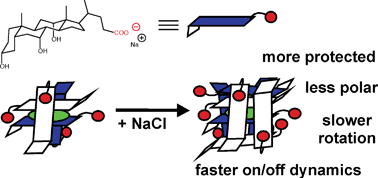
- This article is part of the themed collection: In honour of the contribution of Japanese scientists to photochemistry

 Please wait while we load your content...
Please wait while we load your content...