Tetra- and tri-thienyl ethenes: new fluorescent photochromic compounds
Abstract
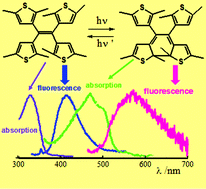
In this work the photochemistry and photophysics of two new ethenic compounds, a tetra-thienylethene (tetrakis) and a tri-thienylethene (triakis), have been investigated by steady state and time-resolved UV-vis absorption and luminescence spectroscopies. The quantum yields of the UV-photoinduced ring-closure reaction (ΦO→C) and of the visible-stimulated cycloreversion reaction (ΦC→O) were determined as a function of the temperature in
 Please wait while we load your content...
Please wait while we load your content...