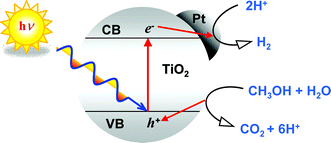
The effect of the crystalline phase of TiO2 (anatase, rutile and brookite) on its photocatalytic activity in hydrogen production from methanol–water vapours has been investigated by testing a series of both home-made and commercial TiO2 photocatalysts, either bare or surface-modified by deposition of a fixed amount, i.e. 1 wt%, of platinum as co-catalyst. For all of the TiO2 samples the rate of hydrogen production increased by one order of magnitude upon Pt deposition, because of the ability of Pt to enhance the separation of photoproduced electron–hole pairs. Under irradiation in the 350–450 nm wavelength range, brookite and anatase showed similar photoactivities, both superior to that of rutile. By contrast, rutile, possessing a narrower band gap, was active also under visible light (λ > 400 nm), whereas no hydrogen evolution was observed with anatase and brookite under such conditions. Surface area proved to be a key parameter, strongly influencing photoactivity. However, as the particle size became ultra-small, the semiconductor absorption edge was blue-shifted because of size quantisation effects, with a consequent decrease in hydrogen production rate due to the smaller portion of incident photons absorbed by the photocatalyst.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...