Radical arylation of tyrosine and its application in the synthesis of a highly selective neurotensin receptor 2 ligand†‡
Abstract
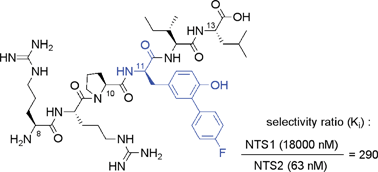
A small library of Fmoc-protected 3-arylated tyrosines was created by radical
- This article is part of the themed collection: Free Radical Chemistry special themed issue in memory of Athel Beckwith

 Please wait while we load your content...
Please wait while we load your content...