CF2-Containing acetylenephosphonates in heterocyclization reactions: the first synthesis of 2-difluoromethyl azaxanth-3-ylphosphonates†
Abstract
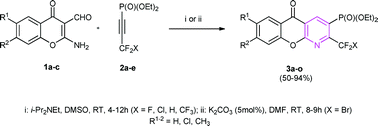
Acetylenephosphonates carrying the XCF2 group have been studied in a base-mediated heterocyclization reaction with selected 2-amino-3-formylchromones to give 2-difluoromethyl azaxanth-3-ylphosphonates. The presence of the fluorinated substituent determined the regioselectivity as well as the reactivity of this process.

 Please wait while we load your content...
Please wait while we load your content...