PtI2-Catalyzed tandem 3,3-rearrangement/Nazarov reaction of arylpropargylic esters: synthesis of indanone derivatives†
Abstract
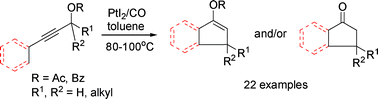
An efficient PtI2-catalyzed tandem reaction of arylpropargylic esters, involving 3,3-rearrangement and Nazarov reaction, has been developed to produce 3-substituted and 3,3-disubstituted

 Please wait while we load your content...
Please wait while we load your content...