Total synthesis of the proposed structures of the DNA methyl transferase inhibitors peyssonenynes, and structural revision of peyssonenyne B†
Abstract
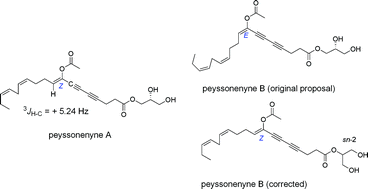
The purported structures of the peyssonenynes A and B isolated from Peyssonnelia caulifera, and considered to be geometric isomers at the acetoxyenediyne moiety, have been synthesized. The E and Z geometries of the synthetic compounds were secured by the magnitude of the 3JH9–C7 values measured using the EXSIDE band-variant of the gradient HSQC

 Please wait while we load your content...
Please wait while we load your content...