Regio- and stereoselective synthesis of truncated 3′-aminocarbanucleosides and their binding affinity at the A3adenosinereceptor
Abstract
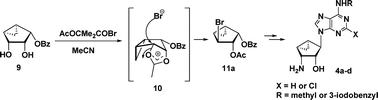
The stereoselective synthesis of truncated 3′-aminocarbanucleosides 4a–dvia a stereo- and regioselective conversion of a

 Please wait while we load your content...
Please wait while we load your content...