Highly diastereoselective vinylogous Mukaiyama aldol reaction of α-keto phosphonates with 2-(trimethylsilyloxy)furan catalyzed by Cu(OTf)2†
Abstract
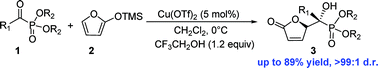
The diastereospecific formation of δ-hydroxyalkylbutenolide phosphonate has been achieved via a vinylogous Mukaiyama aldol reaction. The reaction was performed using α-ketophosphonate 1 and 2-(trimethylsilyloxy)furan 2 mediated by Cu(OTf)2 and
 Please wait while we load your content...
Please wait while we load your content...