C,C-Diacetylenic phosphaalkenes in palladium-catalyzed cross-coupling reactions†
Abstract
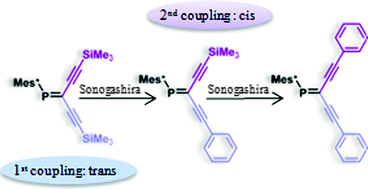
The reactivity of bis-TMS-substituted C,C-diacetylenic phosphaalkene (A2PA) 1 in Sonogashira–Hagihara cross-coupling reactions has been examined. The selective and successive deprotection of the two silyl groups in 1 is enabled by the steric bulk of the Mes* group which renders the acetylenetrans to Mes* more reactive and thereby facilitates selective and consecutive couplings with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is triggered by a
C bond is triggered by a

 Please wait while we load your content...
Please wait while we load your content...