Synthesis and characterization of bis-cyclopropanated 1,3,5-tricarbonyl compounds. A combined synthetic, spectroscopic and theoretical study†
Abstract
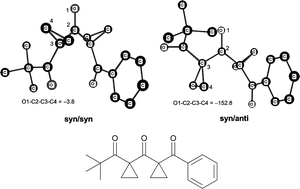
Bis-cyclopropanated 1,3,5-tricarbonyl compounds were prepared by a sequence of Claisen condensations and cyclopropanations. The optimization of the conditions proved to be very important to suppress retro-Claisen reactions. The conformation of these molecules was studied by experimental and computational methods. The syn/syn;syn/syn conformation is present for all derivatives. It is exclusively present in the case of the derivative containing a
 Please wait while we load your content...
Please wait while we load your content...