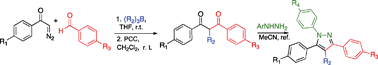
Base-free two-step synthesis of 1,3-diketones and β-ketoesters from α-diazocarbonyl compounds, trialkylboranes, and aromatic aldehydes†‡
Abstract
We describe a convergent, base-free two-step synthesis of 1,3-diketones and β-ketoesters from α-diazocarbonyl compounds, trialkylboranes, and aromatic
 Please wait while we load your content...
Please wait while we load your content...