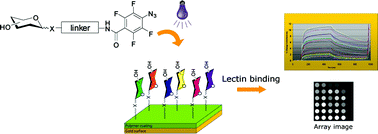
A series of light-activatable perfluorophenylazide (PFPA)-conjugated carbohydrate structures have been synthesized and applied to glycoarray fabrication. The glycoconjugates were structurally varied with respect to anomeric attachment, S-, and O-linked carbohydrates, respectively, as well as linker structure and length. Efficient stereoselective synthetic routes were developed, leading to the formation of the PFPA-conjugated structures in good yields over few steps. The use of glycosyl thiols as donors proved especially efficient and provided the final compounds in up to 70% total yield with high anomeric purities. PFPA-based photochemistry was subsequently used to generate carbohydrate arrays on a polymeric surface, and surface plasmon resonance imaging (SPRi) was applied for evaluation of carbohydrate-protein interactions using the plant lectin Concanavalin A (Con A) as a probe. The results indicate better performance and equal efficiency of S- and O-linked structures with intermediate linker length.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...