Sequential coupling/desilylation–coupling/cyclization in a single pot under Pd/C–Cu catalysis: Synthesis of 2-(hetero)aryl indoles†‡
Abstract
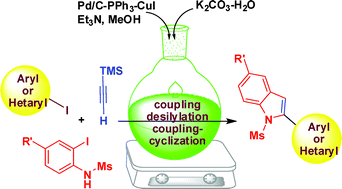
A new one-pot synthesis of 2-(hetero)aryl indolesvia sequential C–C coupling followed by C–Si bond cleavage and a subsequent tandem C–C/C–N bond forming reaction is described. A variety of functionalized

 Please wait while we load your content...
Please wait while we load your content...